Important information for General Practitioners who care for pregnant women
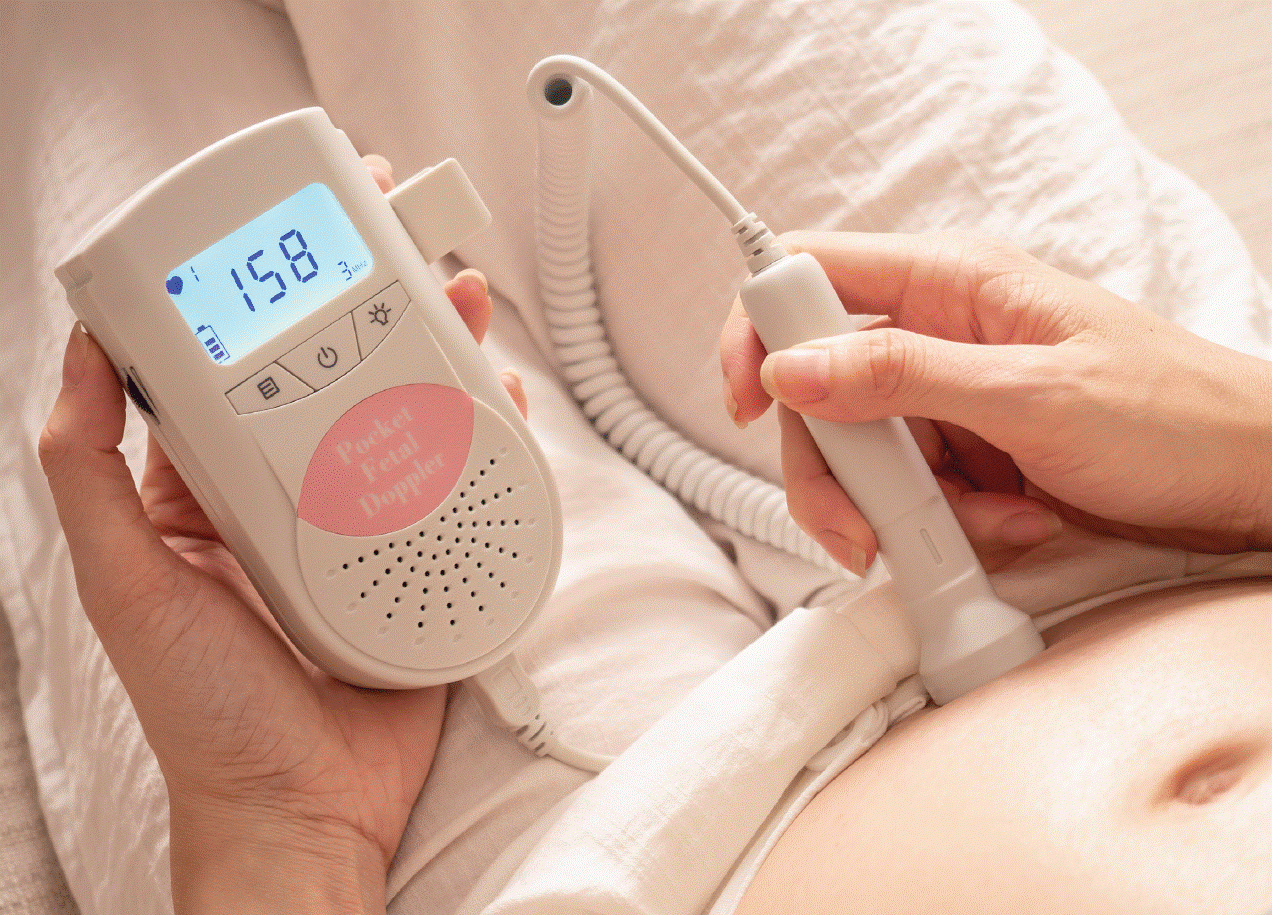
An important update on the use of home fetal heart monitors (dopplers)
An audit in 2021-2022 conducted by the Queensland Maternal and Perinatal Quality Council. The audit uncovered potential risks associated with the use of home fetal heart monitors in pregnancy. The audit identified 4 clinical incidents involving the use of fetal dopplers at home, which saw women, concerned about a lack of fetal movement, being falsely reassured about their baby’s heart rate. The clinical incidents included three stillbirths and one baby dying shortly after birth.
In response to the reports, the TGA undertook a post-market review of all home use dopplers included in the Australian Register of Therapeutic Goods (ARTG). The purpose was to determine whether the risk of using these devices outweighed the potential benefit.
On September 4, 2024, the TGA published the findings of a post-market review of all home use dopplers included in the Australian Register of Therapeutic Goods (ARTG).
They announced: “All home-use fetal dopplers that were intended to be used without the supervision of a healthcare professional have been removed from the ARTG.” The cancellation means home-use devices will no longer be available for purchase. The TGA also considers Baby Movement Apps to be medical devices and must be included in the ARTG. These digital products may deter people from seeking medical attention if they are concerned about their baby’s well-being.
Please review the information provided by the Queensland Government about how GPs can assist with this issue. Practices can also display the poster, warning women about the dangers of relying on home fetal dopplers to monitor their baby's heartbeat.


